
BEAULAURIER'S
SCORE
PROPOSAL
eRA Commons Connect:
https://commons.era.nih.gov/commons/
eRA Commons Help Desk
Phone: 301-402-7469 or 866-504-9552 (Toll Free)
Business hours M-F 7:00 a.m. – 8:00 p.m. Eastern Time
Email commons@od.nih.gov
FIU MBRS Website:
www.fiu.edu/~mbrs
Important Notices:
Look at OSRA web page for new IDC rates.
(Note: HIV project could go for expedited review September 7, 2010)
| SUMMER 2010 | STATUS | ACTIVITY |
| ??? |
|
|
| ??? |
|
|
| ??? |
|
|
| ??? |
|
|
| ??? |
|
|
| ??? |
|
|
| ??? |
|
|
| Sept. 8 |
|
|
| Sept. 14 |
|
|
| Sept. 25 |
|
|
| Sept. 26 |
|
|
| ??? |
|
|
|
??? |
|
|
|
??? |
|
|
|
??? |
|
|
Fillable Individual PHS 398 Forms
THESE FORMS CAN BE FOUND HERE Beaulaurier's document locations:
|
||
| FOR SF424 see PDF files | Document
Name and colors:
|
|
| FIU Forms (IP & CP) | ||
| FIU Budget Forms | ||
| SF424 in |
Comments in:
oppPAR-08-027-cfda93.859-cidADOBE-FORMS-B.pdf |
|
| SF424 (R&R) |
Filled out form Additiona_congressiona_districts.pdf |
complete |
| Project/Performance Site Locations* | Filled out form | complete |
| Research & Related Other Project Info |
Requires Following Attachments:
|
|
| Research & Related Senior/Key Person Profile* |
developmental stuff from 2nd
attempt.doc bio_Beaulaurier_2010_0819.doc bio_Tubman_2010 0819.doc bio_Tashakkori_bio_2010_0818.doc bio_gil_2010_0824.doc |
|
| PHS 398 Cover Page Supplement | ||
| PHS 398 Modular Budget | ||
| PHS 398 Research Plan |
01 Introduction to the
Application 02_specific_aims.doc 03 Research Strategy 04 Inclusion Enrollment Report |
|
| PHS 398 Checklist | ||
| PHS Cover Letter Re. NIA interest | ||
| SF424 Adobe Forms-B document submitted to grants.gov | ||
| Application downloaded from eRA Commons | ||
| Summary Sheets | ||
* Change in name or order of forms from 2nd
submission
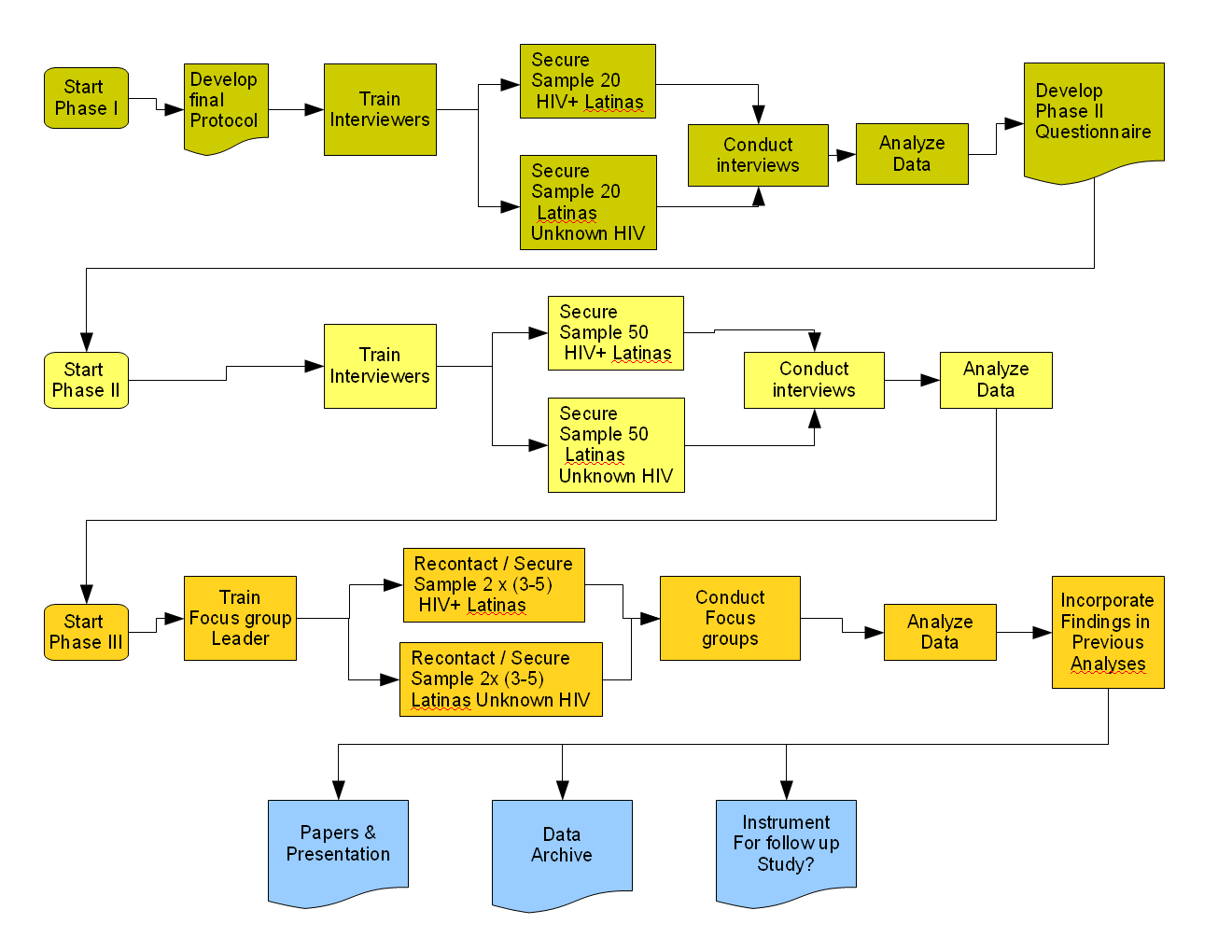
Graphic
below needs updating...
...All HIV+/STD+ respondents?...
...Need more respondents in Phase II?...
*What College/School to assign this to:
MBRS/BRTA
Department: MBRS/BRTA
Dept ID: 124401
Contact Name: Alberto Rivera-Rentas, Ph.D.
Street Address (etc.): NIH BLg45 Rm AS.37 MSC 6200, Bethesda, MD 20892
Phone: 301-594-3900; Fax: 301-480-2753; Web: riverara@nigms.nih.gov
FIU Contacts...
| Name: GISSELLE CASTANEDA Title: SR.SECRETARY Dept: MBRS / MINORITY BIOMEDICAL RESEARCH Office: HLS2 391 Phone: 305-348-1967 Fax: 305-348-7624 grcastan@fiu.edu |
Name: C. H. BIGGER Title: PROFESSOR; DIRECTOR Dept: BIOL. SCI.; MBRS PROGRAMS Office: HLS 319C Phone: 305-348-3100 Secondary Phone: 305-348-1967 Fax: 305-348-1986 biggerc@fiu.edu |
Name: AILEEN LANDRY Title: RESEARCH COORDINATOR MBRS Dept: MBRS Office: HLS II 397 Phone: 305-348-7311 Fax: 305-348-7624 Aileen.Landry@fiu.edu |
SC2 PAR-08-027 http://grants.nih.gov/grants/guide/pa-files/PAR-08-027.html
NIGMS MBRS Website http://www.nigms.nih.gov/Minority/MBRS/default.htm
SCORE FAQ: http://www.nigms.nih.gov/Minority/MBRS/SCOREUpdateFAQ.htm
SF424 (R&R)
Application Package and Guide (includes directions)
http://www.grants.gov/applicants/apply_for_grants.jsp
App info as of 5/20/2010:
| CFDA | Opportunity Number | Competition ID | Competition Title | Agency | |
|---|---|---|---|---|---|
| 93.859 | PAR-08-027 | ADOBE-FORMS-B | Adobe-Forms-B | National Institutes of Health |
closing date: 09/07/2011
SPECIAL INSTRUCTIONS
Applicants are advised to follow carefully the instructions given for electronic submission and the use of the SF424 (R&R) form at http://era.nih.gov/ElectronicReceipt/. Below are special instructions for this FOA that describe the information that must be included in the required components mentioned above. Incomplete or non-compliant applications will be withdrawn and will not be reviewed.
a. The total student enrollment at the institution and
the number and percent of underrepresented minorities (e.g., Native American,
African American, Hispanic American, natives of the U.S. Pacific Islands) in the
total student population;
b. Number and percent of underrepresented minority students (undergraduate and
graduate) enrolled in the sciences relevant to biomedical and behavioral
research;
c. Number and percent of total and underrepresented minority faculty in the
sciences relevant to biomedical and behavioral research and the total number of
faculty and of underrepresented minority faculty from these departments
participating in funded research.
Preliminary Studies for New Applications and Progress Reports for Renewal and Revision Applications - any PI who has previously received SCORE support (under an SC or an earlier S06 subproject) must include a progress report indicating the extent to which the proposed objectives were accomplished. The Progress Report, which does count toward the Research Strategy page limit, must be clearly indicated in a subheading within the Preliminary Data category. PIs who have had a gap in funding must still include a report of the research accomplishments that they have achieved with previous SCORE support. This subsection is required, even though, for the purposes of electronic submission to Grants.gov, the application must be designated on the face page as New.
The Principal
Investigator’s developmental objectives and plan should be included as part of
the Biographical Sketch within the page limits (4 pages) of the Personal Statement.
The developmental objectives and plan must present how the PIs SC2 and research
career goals will be achieved as a logical progression from the candidates
current stage and past research experience. The plan must justify the need for
development; provide an explanation of how the proposed project and the time
devoted to it will help the PI further his/her research competitiveness; and
describe how the proposed research project guided by a mentor will significantly
improve the PIs productivity and the transition to the next step in his/her
research career. A timeline for the transition to the next stage, i.e., a full
research project, and publication plan must be provided in this section.
The Biographical Sketch for each mentor
is required as part of key personnel and it should include a
description of the
role of the mentor(s) and mentoring plan within the page limits
(4 pages) of the
mentor’s
personal statement. This should provide: (1) information on his/her research
qualifications and previous experience as a research supervisor/mentor; (2) a
mentoring plan that describes the nature of the supervision and mentoring that
will occur during the proposed award period; and (3) a plan for monitoring the
applicant’s research, publications, and transition to the next step in his/her
research career.
Please note that a letter from the mentor(s) is no longer
required.
GRANTS.GOV & ERA COMMONS
Grants submitted electronically through:
http://www.grants.gov
...for help...
Grants.gov/Apply
for Grants &
http://grants.gov/CustomerSupport
Grants.gov Customer
Support
Contact Center Phone: 800-518-4726
Business Hours: M-F 7:00 a.m. - 9:00 p.m. Eastern Time
Email support@grants.gov
DATES:
Application Submission Dates: Standard dates for SC2 applications apply,
please see
http://grants1.nih.gov/grants/funding/submissionschedule.htm for details.
Peer Review Date(s): Standard dates apply, please see
http://grants1.nih.gov/grants/funding/submissionschedule.htm#reviewandaward
for guidance on dates.
Council Review Date(s): Standard dates apply, please see
http://grants1.nih.gov/grants/funding/submissionschedule.htm#reviewandaward
for guidance on dates.
Earliest Anticipated Start Date: Standard dates apply, please see
http://grants1.nih.gov/grants/funding/submissionschedule.htm#reviewandaward
for guidance on dates.
MISC INFO:
More resources:
Rich's Obscure but Useful References